.avif)
Did you know that a beauty product’s success isn’t just about how it looks or smells, but how consistently it performs?
Behind every glowing review and repeat purchase lies one crucial factor: quality control.
For beauty entrepreneurs, mastering quality control means more than just passing tests. It’s about ensuring that each serum, lotion, or cleanser feels exactly the same from the first batch to the hundredth, safe, stable, and effective.
Let’s take a closer look at how beauty product quality control shapes everything from your first formulation to your final shelf-ready product.
Everything You Need to Know About Cosmetic Products Quality Control
In simple terms, cosmetics quality control is the process of checking that your cosmetics meet safety, consistency, and performance standards before they reach your customers.
It’s part of a larger quality system that includes both quality assurance (the overall process of maintaining standards) and quality control (the actual testing and verification).
Think of it like the final checkpoint before your product meets the world, where every ingredient, texture, scent, and color is examined to ensure they all live up to your brand’s promise.
For private label skincare brands or companies targeting other branches of the beauty industry, this process often involves working with manufacturing partners who already have built-in cosmetics formulation and manufacturing quality control systems in place.
This ensures that your product is safe, compliant, and consistent, no matter how large your production run becomes.
Why Quality Control Matters for Your Brand
Quality control isn’t just a formality; it’s the foundation of your brand’s reputation.
If you are creating your own skincare line or makeup brand, rest assured that the consumers will trust that your moisturizer won’t separate, your serum won’t oxidize, and your cleanser won’t irritate their skin.
Without strict quality control measures, even a well-formulated product can fail. Issues like microbial growth, pH imbalance, or unstable ingredients can quickly lead to product recalls, poor reviews, and loss of trust.
Strong QC systems also:
- Ensure regulatory compliance in every market
- Maintain consistent texture, fragrance, and performance
- Extend product shelf life
- Prevent contamination and spoilage
- Build credibility for long-term customer loyalty
Many people selling private label cosmetics maintain relationships with trusted manufacturers and testing laboratories and can facilitate testing as part of their service offering.
Key Stages of Quality Control in Cosmetics Manufacturing
Every beauty product goes through several quality control stages before it’s cleared for sale. Here’s what typically happens behind the scenes:
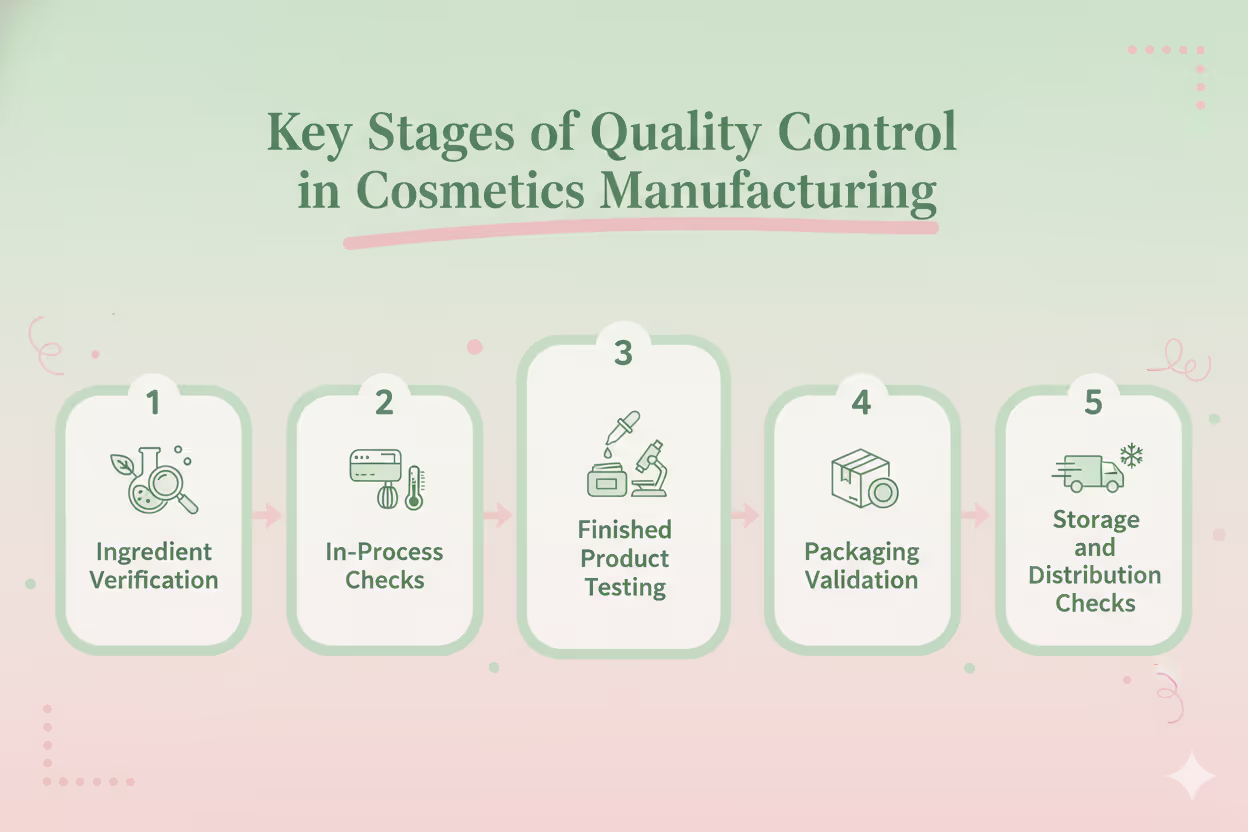
1. Ingredient Verification
Before production even begins, raw materials are tested for purity, potency, and compliance with safety regulations.
This ensures that only approved, high-quality ingredients enter your formulation, minimizing the risks of irritation or contamination.
2. In-Process Checks
During production, QC teams monitor each step to confirm consistency in pH, viscosity, color, and odor.
If something falls out of range, it’s corrected immediately. This prevents wasted batches and ensures reliable results.
3. Finished Product Testing
Once your formulation is complete, samples are tested for microbial safety, stability, and physical consistency.
No product leaves the lab until it passes these essential benchmarks.
4. Packaging Validation
Choosing the right packaging for skincare and beauty products plays a huge role in maintaining product quality. Tests confirm that bottles, pumps, or jars don’t react with the formula or compromise its shelf life.
5. Storage and Distribution Checks
Finally, products are monitored for how they perform under different conditions. This includes temperature changes and shipping stress, ensuring that they reach your customers in perfect condition.
By the time your beauty product lands in a customer’s hands, it’s already passed multiple layers of cosmetics quality control designed to protect both your brand and your buyers.
Testing Methods Behind Cosmetic Quality Control
Let’s dive into some of the key tests that keep your beauty products safe, stable, and effective.

Microbiological Testing
No one wants bacteria in their beauty products. Microbiological testing checks for harmful microorganisms that could cause irritation or spoilage.
Tests often include:
- Total Aerobic Microbial Count (TAMC)
- Yeast and Mold Count (TYMC)
- Pathogen testing for bacteria like Staphylococcus aureus or Pseudomonas aeruginosa
For water-based products, preservative efficacy testing ensures your formula stays protected against microbial growth over time.
Stability Testing
Ever noticed how some products change color or texture over months? That’s where stability testing comes in.
Products are stored at different temperatures, light exposures, and humidity levels to simulate real-life conditions. This helps determine:
- Shelf life
- Ideal storage temperatures
- Packaging compatibility
Compatibility Testing
Packaging and formulation need to get along. This test ensures that your container doesn’t cause ingredient separation, leakage, or oxidation.
Claim Validation (Efficacy Testing)
If your product claims to “hydrate for 24 hours” or “improve skin elasticity,” efficacy testing provides proof.
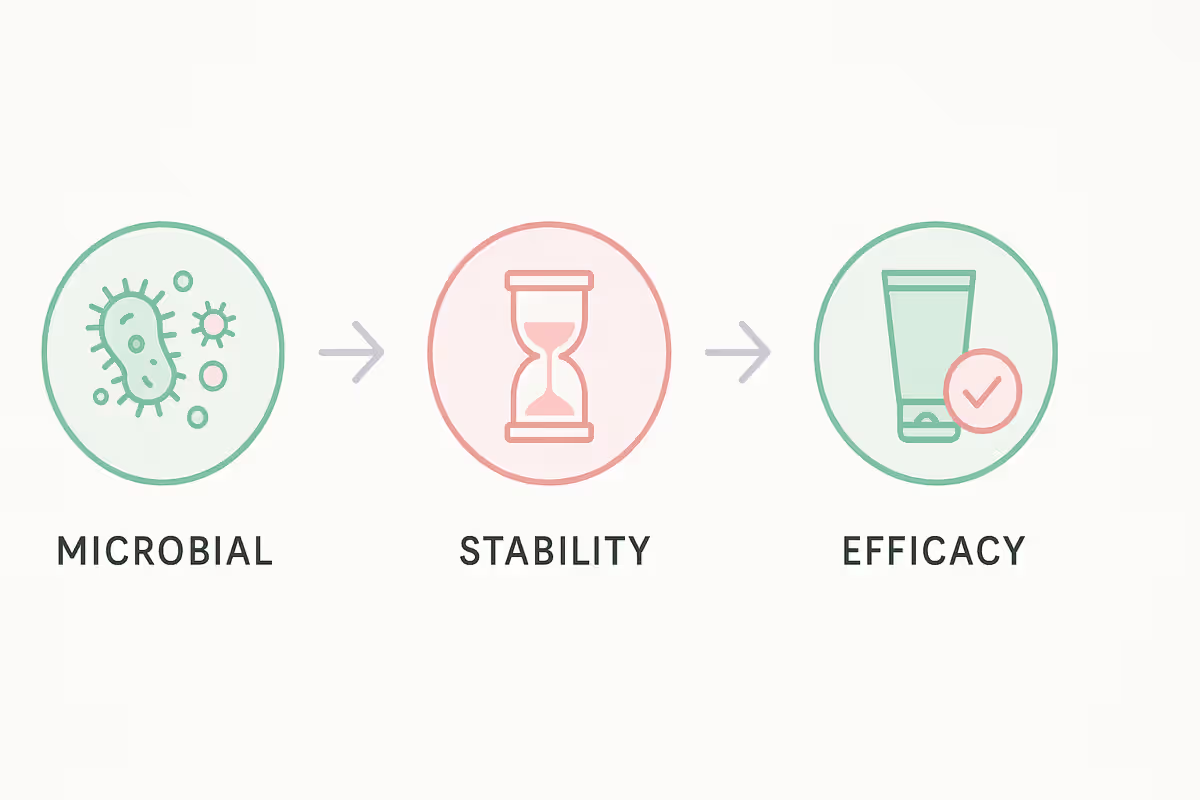
This includes both in-vitro (lab-based) and in-vivo (on human volunteers) methods to validate those results.
By combining these tests, brands can confidently say their products do exactly what they promise.
Meeting Global Compliance Standards
If your beauty brand sells internationally, understanding global compliance is essential.
In the U.S.
The FDA doesn’t pre-approve cosmetics but expects brands to ensure their products are safe for consumer use. Manufacturers must follow Good Manufacturing Practices (GMP) and maintain accurate ingredient documentation.
In the European Union
The EU has stricter rules under Regulation (EC) No. 1223/2009, requiring safety assessments, stability testing, and a Product Information File (PIF) for every item sold.
Other Markets
- China: Often requires mandatory animal testing for imported cosmetics.
- Japan: Enforces ingredient restrictions and specific documentation standards.
- ASEAN countries: Follow harmonized guidelines similar to the EU.
Working with a manufacturer like Supliful ensures these complex compliance steps are already handled. This allows you to focus on branding and your beauty product marketing instead of legal paperwork.
Common Quality Control Challenges
Even with the best systems in place, challenges can arise during cosmetics formulation, manufacturing quality control.
Ingredient Variability
Natural ingredients can vary by source or season, affecting texture and color. Careful supplier selection and testing help maintain consistency.
Packaging Interactions
Sometimes, the packaging affects the formula, especially with active ingredients or essential oils. Compatibility testing catches these issues early.
Batch Consistency
Every batch must match the original standard. Continuous in-process checks help prevent slight differences in viscosity or scent.
Testing Reproducibility
Different labs or testing methods can yield slightly different results. That’s why working with accredited labs that follow ISO standards is so important.
How Supliful Simplifies the Quality Control Process
At Supliful, cosmetic product quality control isn’t an afterthought. It’s part of every step in the production journey.
Here’s how Supliful helps founders bring top-quality products to market with confidence:
- Pre-tested formulations: Every Supliful formula goes through microbiological, stability, and performance testing before it’s made available to creators.
- Certified laboratories: Testing is performed in accredited facilities following GMP and ISO 22716 standards.
- Transparent data: Brands can access detailed testing reports and documentation for compliance purposes.
- Consistent manufacturing: Every batch follows the same strict QC protocols to ensure uniform texture, color, and efficacy.
With Supliful, you don’t have to navigate the technical side of testing alone. You get built-in support from professionals who understand both formulation science and regulatory requirements.
If you’re considering the profitability of dropshipping your beauty products, be sure to also explore how supplier structure works in this model, as this is the common choice for many brands.
Building a Quality-First Beauty Brand
Strong quality control systems do more than ensure safety. They build credibility, longevity, and consumer trust.
When your products perform consistently and meet regulatory standards, your beauty product customers feel confident coming back for more.
Quality control also helps you:
- Reduce returns and recalls
- Improve customer satisfaction
- Support clean beauty claims with data
- Maintain regulatory compliance as you scale
When quality becomes part of your brand’s DNA, it shows not just in your products, but in every customer experience.
Build a Brand Customers Trust with the Right Control System in Place
Quality control might sound like a behind-the-scenes process, especially if you’re only now creating a beauty brand, but it’s truly the foundation of your brand’s success.
From verifying ingredients to validating claims, each test ensures your products are safe, effective, and reliable, the very things customers value most.
If you’re ready to launch or scale your beauty line with confidence, Supliful’s built-in quality systems make it easier than ever. From formulation to final packaging, you’ll know your products meet the highest standards before they ever reach the shelf.
Turn your product idea into a brand that customers trust with Supliful. Get in touch now!
The information provided in this article is meant for general informational purposes only and should not be considered as professional or legal advice. We do not guarantee the completeness, accuracy, reliability, or suitability of the information in this article. We strongly recommend seeking professional guidance that suits your individual circumstances.
FAQ
Related blogs
.avif)
Stay Ahead of the Curve: Top Supplement Trends to Watch and Sell